-
About
- Departments & Offices
-
Academics
- Public Health
- Biomedical Sciences
- Physician Assistant
- Special Master’s (MBS)
-
Admissions & Financial Aid
- Tuition & Fees
-
Student Experience
-
- Student Resources by Program
- Academic & Student Support
- Wellness & Wellbeing
- Student Life
- Events & Traditions
-
-
Research
- Research Labs & Centers
- Tufts University-Tufts Medicine Research Enterprise
-
Local & Global Engagement
- Global Health Programs
- Community Engagement
Cultured stem cells reconstruct sensory nerve and tissue structure in the nose
Lab-grown olfactory stem cells will enable research into therapies for recovering sense of smell after injury or degeneration
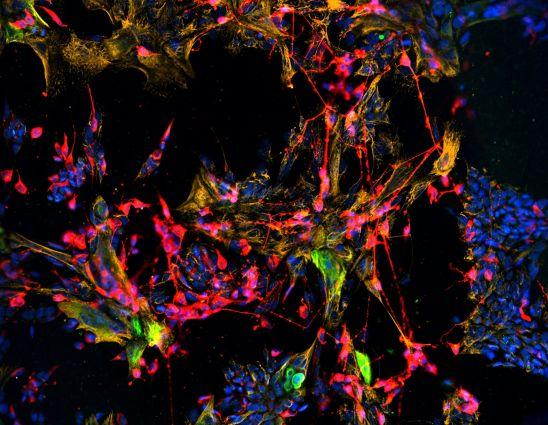
BOSTON (March 28, 2019, 11:00 a.m. ET)—A team of researchers at Tufts University School of Medicine developed a method to grow and maintain olfactory stem cells in culture, which can then be used to restore tissue in the nose. The discovery raises hope that future therapies could be developed to restore the sense of smell in individuals where it has been damaged by injury or degeneration.
The stem cells, called horizontal basal cells (HBCs), can repopulate all olfactory epithelium (OE) cell types, including sensory neurons, when transplanted into injured tissue. Published today in the journal Stem Cell Reports, the development paves the way for further research into stem cell transplantation therapies, or pharmacological approaches that stimulate stem cells within the nose to regenerate tissue.
The nerves that confer the sense of smell are unique when compared to the rest of the nervous system, in that they can trigger a robust and nearly complete regenerative response after injury. OE tissue contains two types of stem cells – globose basal cells (GBCs) and HBCs. The GBCs have been successfully cultured and appear to have a primary role in repopulating cells that have been lost to routine turnover. HBCs, however, remain dormant and are not activated until there is an injury. Unfortunately, studies on these cells have been limited by the fact that they could not be expanded and maintained in culture. In this study, the researchers determined the optimal conditions for expanding and maintaining healthy HBC stem cells in culture, borrowing methods and factors used to maintain respiratory stem cells.
“Once we determined that we could grow HBCs in the lab, and that they expressed the same identifying molecular markers found in vivo, we sought to confirm whether they would work as well as the in vivo HBCs – can they regenerate tissue that has been injured – and they did!” said James Schwob, M.D., Ph.D., professor of developmental molecular and chemical biology at Tufts University School of Medicine, and corresponding author for the study.
Despite a natural ability for regeneration, dysfunction in the sense of smell is still reported among 19.4 percent of the population (OLFACAT study, 2003), with 0.3 percent experiencing complete loss of smell. Causes range from aging, injury, smoking, and neurodegenerative diseases to certain medications.
Schwob and his team discovered that the lab-grown HBCs were able to fill in olfactory lesions, generating multiple cell types including Sus cells, basal cells and olfactory sensory neurons.
“The HBCs in culture remained quiescent, pretty much as they do in vivo, but we were able to trigger them into an active state to start the process of differentiation into various olfactory epithelial cells just before engrafting them into injured tissue,” said Jesse Peterson, Ph.D., first author of the study and currently a post-doctoral fellow at the MRC Laboratory of Molecular Biology. Peterson conducted the study as part of his doctoral dissertation at the Sackler School of Graduate Biomedical Sciences at Tufts, advised by Schwob.
The trigger used was retinoic acid, which has the effect of lowering levels of the protein P63 in the cells, leading to stem cell activation. P63 functions as a “master control switch” and is known to decrease levels during injury, transitioning HBCs from dormancy to activation in vivo. A more thorough understanding of the role of P63 has been hampered by the slow pace of in vivo studies. With lab-grown HBCs, the mechanisms of activation can be examined more closely.
“Now that we can create a reserve of dormant stem cells, we see this as a useful tool for exploring ways to guide cell differentiation toward specific cell types, and develop new stem cell therapies for tissue and sensory regeneration – using the patient’s own stem cells for culturing and transplantation, or pharmacological interventions to activate the patient’s own dormant stem cells within the nose,” said Schwob.
Other authors contributing to this study are Brian Lin, Ph.D., Camila Barrios-Camacho, Daniel Herrick, M.D., Ph.D., and Julie Coleman, Ph.D., all graduates or students at Tufts, Woochan Jang, Ph.D., former research assistant professor at Tufts, and Eric Holbrook, M.D., of Massachusetts Eye and Ear, and Harvard Medical School.
This work was supported by awards from the National Institutes of Health’s National Institute on Deafness and Other Communication Disorders (R01DC002167, F31DC014637, F30DC013962, F31DC014398). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Peterson, J., Lin, B., Barrios-Camacho, C.M., Herrick, D., Holbrook, E.H., Jang, W., Coleman, J.H. and Schwob, J.E., "Activating a reserve neural stem cell population in vitro enables engraftment and multipotency after transplantation," Stem Cell Reports (2019). DOI: 10.1016/j.stemcr.2019.02.014
Department:
Developmental Molecular and Chemical Biology