-
About
-
Academics
- Physician Assistant
- Special Master’s (MBS)
-
Admissions & Financial Aid
- Tuition & Fees
-
Student Life
-
Research
- Research Labs & Centers
-
Local & Global Engagement
- Global Health Program
Stem Cells and Silk Make a New Way to Study the Brain
An NIH grant powers collaborative research on Alzheimer’s disease
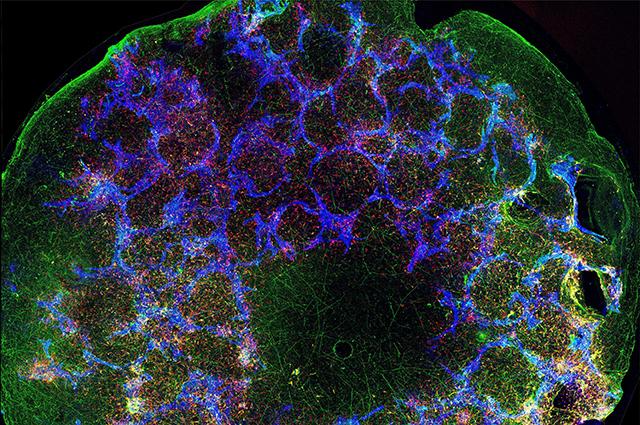
More than five million Americans, mostly sixty-five or older, suffer from Alzheimer’s disease (AD), and that number is expected to triple by 2060, as today’s twenty-somethings become seniors. No treatments exist for this devastating disease, and its root causes remain as tangled as the curious brain deformities that German physician Alois Alzheimer first described in 1906.
Now a team of Tufts researchers from the School of Medicine and the School of Engineering has received a five-year, $5 million grant from the National Institute on Aging, part of the National Institutes of Health, to study the role of different cell types and mutations in AD. They will use a unique bioengineered “mini brain” that realistically simulates the human brain environment for years.
The work, which builds on years of collaboration among the researchers, will overcome two traditional stumbling blocks to such studies: the limited relevance of animal models and the inability of cell culture systems to reproduce the physiology of the human brain. While age is the biggest risk factor for AD, genetics also plays a role. Scientists have uncovered twenty gene variants that increase the risk of AD, said Giuseppina Tesco, professor of neuroscience and lead investigator on the research, who has devoted her career to studying the disease.
Recent studies show that most of the genes that carry these variants are expressed in glial cells, particularly astrocytes and microglial cells. Once dismissed as onlookers in the brain, glia are now “front and center” in Alzheimer’s research said glia expert Philip Haydon, a principal investigator on the project. Haydon, the Annetta and Gustav Grisard Professor of Neuroscience, likens these cells to the pit crew for the flashy race-car-like neurons, supporting top performance by, for example, preventing buildup of protein plaques.
But unlike neurons, human glial cells behave very differently from those of other mammals. “What we can learn from mouse models is very limited. It is very important to study these genes in human cells,” said Tesco. “And we need to do this over time. It may take months to see the effect of genetic variation.”
The Tufts team will use cells derived from patients with AD as well as healthy subjects, drawing on advanced stem cell technology that makes it possible to “reverse engineer” human primary cells into induced pluripotent stem cells, which can then differentiate into neurons, astrocytes, and microglia.
These glia and other brain cells will grow on a unique three-dimensional doughnut-shaped scaffold made of porous silk and collagen—what the researchers have dubbed a “mini brain.” Bioengineer David Kaplan, Stern Family Professor and a principal investigator on the grant, and his team have spent six years perfecting the mini brain for research on AD, traumatic brain injury, and brain cancer.
“This model allows us to put cells where we want, determine ratios of different cells to use in the system, and control interactions, so we can study electrophysiology, synaptic activity, and other functions as the tissue ages,” said Kaplan. That control over the long term supports exploration of age-related questions about disease progression and contributes to reproducibility, a scientific pillar. Past experiments using these mini brains have mimicked structural and functional features and neural activity for up to two years.
In contrast, a two-dimensional culture system—like the proverbial petri dish—won’t replicate the complexities of multiple cell types and physiologies. And organoids—simplified organs in miniature now in vogue—are subject to cellular death after a few weeks or months.
To complement the in vitro studies with the scaffolds, scientists in Haydon’s lab will transplant some of the human cells, both mutated and normal, into mice. As they grow, the human glia cells will replace the mouse cells, giving researchers an opportunity to study human brain function. “This is the first step towards translational studies,” said Haydon.
The grant complements donations from Tufts alumni, parents, friends, and other private individuals who have experienced the pain of Alzheimer’s disease in their own lives. “Donor dollars really got some of our early, exploratory work up and running,” said Haydon. “Now we are building on that.”
The NIH support is a bright spot at a time when COVID-19 has forced Tufts scientists, like their peers around the world, to halt laboratory research, sometimes losing years of work.
Tesco said that while it is difficult to be away from her lab, safety is more important than anything else. “I’m from Italy, where we have more than 22,000 deaths,” she said. “Being healthy and having the possibility to continue to do some work, I feel lucky. We’ll be in the best position possible when we’re ready to start because we’ll be able to start something completely new and very exciting.”
Department:
Neuroscience